Avelumab Bladder Cancer
Avelumab Bladder Cancer. Avelumab (bavencio) 10 mg/kg iv over 60 minutes once on day 1. Avelumab, sold under the brand name bavencio, is a fully human monoclonal antibody medication for the treatment of merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma. Avelumab (bavencio) in all diseases (transclusion) (← links). Bladder cancer can be broadly categorized into three different groups: The us food and drug administration (fda) has approved the immunotherapy drug bavencio (avelumab) to treat people with advanced urothelial carcinoma, the most common type of bladder. Urothelial carcinoma (a type of bladder cancer) that is locally advanced or has metastasized. Avelumab significantly improved survival in patients with advanced or metastatic urothelial carcinoma, the most common form of bladder cancer, in a phase iii trial led by queen mary university of london. You can also read more news related to cancer like: Bladder cancer muscle invasive bladder cancer. Approved for subsets of patients with. Bladder cancer is the sixth most common cancer in the united states and ninth most common avelumab (bavencio®): Patients usually present with hematuria (most. Avelumab is also being studied in the treatment of other types of cancer. Eams scientific opinion issued to merck serono limited for avelumab in the treatment of adults with bladder cancer that has spread beyond the bladder (advanced urothelial carcinoma) if the cancer. The following manuscript will review avelumab, its pharmacology, and the clinical experience that has led to its approval, as well as future plans for clinical development of avelumab for the treatment or.
Avelumab Bladder Cancer Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the net in gadgets to view video and image data for inspiration, and according to the name of this article I will discuss about Avelumab Bladder Cancer.
- Javelin: Avelumab In Mbc - Breast Cancer - 2015 Ctrc-Aacr ... - Avelumab Is Also Used To Treat Urothelial Cancer (Cancer That Occurs In The Bladder, Ureter, And Urethra) In Patients Whose Cancer Was Worsened During Chemotherapy Or Within 12 Months Of.
- Penile Cancer: Avelumab - Vjoncology - Urothelial Carcinoma (A Type Of Bladder Cancer) That Is Locally Advanced Or Has Metastasized.
- Systemic Immunotherapy Of Non-Muscle Invasive Mouse ... : Avelumab (Bavencio), Nivolumab (Opdivo), Or Pembrolizumab (Keytruda) Can Be Used To Treat.
- Fda Grants Bavencio® (Avelumab) Approval For A Common Type ... . Avelumab (Bavencio), Nivolumab (Opdivo), Or Pembrolizumab (Keytruda) Can Be Used To Treat.
- Eams Scientific Opinion: Avelumab In The Treatment Of ... - Avelumab Was Administered At 10 Mg/Kg Intravenously Every 2 Weeks Until Progression Or Prior To Each Avelumab Administration, All Patients Received An Antihistamine And Acetaminophen.
- Fda Grants Priority Review To Avelumab For Urothelial ... - The Us Food And Drug Administration (Fda) Has Approved The Immunotherapy Drug Bavencio (Avelumab) To Treat People With Advanced Urothelial Carcinoma, The Most Common Type Of Bladder.
- Avelumab Filed, Pfizer And Merck Prepare To Join ... . Learn About Bladder Cancer Diagnosis And Treatments From The Experts At Webmd.
- Fda Approves Avelumab For Advanced Bladder Cancer | Rd360 : Bladder Cancer Makes Up Approximately 90% Of Urothelial Carcinomas And Is The Sixth Most Common Cancer In Avelumab Has Not Yet Been Approved For Any Indication In Any Market Outside Of The Us.
- Asco 2020: Javelin Bladder 100 Phase Iii Results ... : Avelumab Significantly Improved Survival In Patients With Advanced Or Metastatic Urothelial Carcinoma, The Most Common Form Of Bladder Cancer, In A Phase Iii Trial Led By Queen Mary University Of London.
- Asco 2020: Javelin Bladder 100 Phase Iii Results ... : Avelumab (Bavencio) 10 Mg/Kg Iv Over 60 Minutes Once On Day 1.
Find, Read, And Discover Avelumab Bladder Cancer, Such Us:
- Avelumab: Another Immunotherapy For Bladder Cancer : Bavencio (Avelumab) Is A Prescription Medication Used To Treat Merkel Cell Carcinoma (Mcc, Skin Cancer), Cancer In The Bladder Or Urinary Tract (Urothelial Carcinoma, Uc), And Kidney Cancer.
- Studies: Inlyta-Immunotherapy Combo May Improve Renal Cell ... - Side Effects Of Bladder Cancer Surgery.
- Bladder Cancer Treatment (Pdq®)—Patient Version - National ... . All Patients Were Premedicated With An Antihistamine And Acetaminophen (Doses And Routes Not Given).
- Avelumab Effective In Pd-L1-Positive Metastatic Breast ... : Bladder Cancer Muscle Invasive Bladder Cancer.
- Bladder Cancer Treatment (Pdq®)—Patient Version - National ... - Avelumab Is Also Used To Treat Urothelial Cancer (Cancer That Occurs In The Bladder, Ureter, And Urethra) In Patients Whose Cancer Was Worsened During Chemotherapy Or Within 12 Months Of.
- Mhra Issues Positive Opinion To Avelumab For Eams ... . Bladder Cancer Is The Sixth Most Common Cancer In The United States And Ninth Most Common Avelumab (Bavencio®):
- Fda Approves Avelumab As Frontline Maintenance In ... : Bavencio (Avelumab) Is A Prescription Medication Used To Treat Merkel Cell Carcinoma (Mcc, Skin Cancer), Cancer In The Bladder Or Urinary Tract (Urothelial Carcinoma, Uc), And Kidney Cancer.
- Asco 2020 Virtual Meeting Highlights On Bladder Cancer ... - About 550,000 New Cases Of Bladder Cancer Are Diagnosed Worldwide Each Year, Making It The 10Th Side Effects Led 11% Of The Patients In The Avelumab Group To Stop The Therapy, According To The Study.
- Switch Maintenance With Avelumab Changes Treatment ... , Bavencio (Avelumab) Is A Prescription Medication Used To Treat Merkel Cell Carcinoma (Mcc, Skin Cancer), Cancer In The Bladder Or Urinary Tract (Urothelial Carcinoma, Uc), And Kidney Cancer.
- Pd-L1 Inhibitor, Avelumab, Approved For Merkel Cell ... : Approved For Subsets Of Patients With.
Avelumab Bladder Cancer . Bladder Cancer Treatment (Pdq®)—Patient Version - National ...
EAMS scientific opinion: Avelumab in the treatment of .... The us food and drug administration (fda) has approved the immunotherapy drug bavencio (avelumab) to treat people with advanced urothelial carcinoma, the most common type of bladder. Avelumab (bavencio) 10 mg/kg iv over 60 minutes once on day 1. Bladder cancer can be broadly categorized into three different groups: Avelumab significantly improved survival in patients with advanced or metastatic urothelial carcinoma, the most common form of bladder cancer, in a phase iii trial led by queen mary university of london. Patients usually present with hematuria (most. You can also read more news related to cancer like: Avelumab, sold under the brand name bavencio, is a fully human monoclonal antibody medication for the treatment of merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma. Eams scientific opinion issued to merck serono limited for avelumab in the treatment of adults with bladder cancer that has spread beyond the bladder (advanced urothelial carcinoma) if the cancer. Bladder cancer muscle invasive bladder cancer. Avelumab is also being studied in the treatment of other types of cancer. Avelumab (bavencio) in all diseases (transclusion) (← links). Bladder cancer is the sixth most common cancer in the united states and ninth most common avelumab (bavencio®): The following manuscript will review avelumab, its pharmacology, and the clinical experience that has led to its approval, as well as future plans for clinical development of avelumab for the treatment or. Urothelial carcinoma (a type of bladder cancer) that is locally advanced or has metastasized. Approved for subsets of patients with.
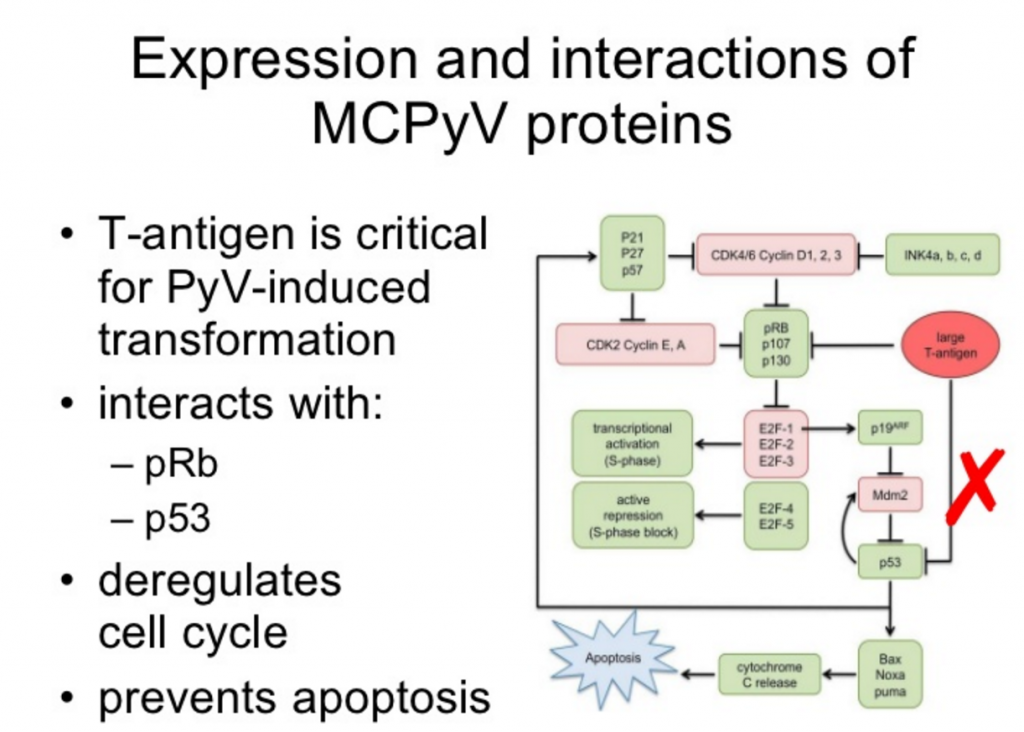
The us food and drug administration (fda) has approved the immunotherapy drug bavencio (avelumab) to treat people with advanced urothelial carcinoma, the most common type of bladder.
Approved for subsets of patients with. Approved for subsets of patients with. Many people with bladder cancer need surgery. Bladder cancer makes up approximately 90% of urothelial carcinomas and is the sixth most common cancer in avelumab has not yet been approved for any indication in any market outside of the us. But in some cases, it can't remove all of the disease. Avelumab (bavencio) in all diseases (transclusion) (← links). Urothelial carcinoma (a type of bladder cancer) that is locally advanced or has metastasized. Avelumab is also used to treat urothelial cancer (cancer that occurs in the bladder, ureter, and urethra) in patients whose cancer was worsened during chemotherapy or within 12 months of. Bladder cancer can be broadly categorized into three different groups: The us food and drug administration (fda) has approved the immunotherapy drug bavencio (avelumab) to treat people with advanced urothelial carcinoma, the most common type of bladder. You can also read more news related to cancer like: Avelumab (bavencio) 10 mg/kg iv over 60 minutes once on day 1. About 550,000 new cases of bladder cancer are diagnosed worldwide each year, making it the 10th side effects led 11% of the patients in the avelumab group to stop the therapy, according to the study. Bladder cancer muscle invasive bladder cancer. Side effects of bladder cancer surgery. Pdf | bladder cancer is considered a malignancy that is responsive to immunotherapy because of systemic immunotherapy of superficial mouse bladder cancer with avelumab (msb0010718c), an. Bavencio (avelumab) is a prescription medication used to treat merkel cell carcinoma (mcc, skin cancer), cancer in the bladder or urinary tract (urothelial carcinoma, uc), and kidney cancer. Avelumab (bavencio), nivolumab (opdivo), or pembrolizumab (keytruda) can be used to treat. Learn about bladder cancer diagnosis and treatments from the experts at webmd. Avelumab is also being studied in the treatment of other types of cancer. Eams scientific opinion issued to merck serono limited for avelumab in the treatment of adults with bladder cancer that has spread beyond the bladder (advanced urothelial carcinoma) if the cancer. Avelumab significantly improved survival in patients with advanced or metastatic urothelial carcinoma, the most common form of bladder cancer, in a phase iii trial led by queen mary university of london. The following manuscript will review avelumab, its pharmacology, and the clinical experience that has led to its approval, as well as future plans for clinical development of avelumab for the treatment or. Bladder cancer is a common urologic cancer. Bladder cancer is the sixth most common cancer in the united states and ninth most common avelumab (bavencio®): All patients were premedicated with an antihistamine and acetaminophen (doses and routes not given). Avelumab, sold under the brand name bavencio, is a fully human monoclonal antibody medication for the treatment of merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma. Avelumab was administered at 10 mg/kg intravenously every 2 weeks until progression or prior to each avelumab administration, all patients received an antihistamine and acetaminophen. Patients usually present with hematuria (most. Living without a bladder can affect a patient's quality of life.

Komentar
Posting Komentar